How does plant nutrient metabolism work?
| Added by:
10k Last edited by:
10k Viewed: 608 times |
Rated by 31 users:
9.50/10 |
Contributed by: aallonharja
Submitted: June 18, 2003 How does plant
nutrient metabolism work? In other words,
how do plants eat? In order to live, plants need
these 16 essential elements, called macronutrients and
micronutrients. Macro Nutrients (primary nutes)
carbon (C)
hydrogen (H)
oxygen (O2)
nitrogen (N)
phosphorus (P)
potassium (K)
calcium (Ca)
magnesium (Mg)
sulfur (S)
Micro Nutrients
(Secondary nutes)
Boron (B)
Chlorine (Cl)
copper (Cu)
iron (Fe)
Manganese (Mn)
Zinc (Zn)
Molybdenum (Mo)
Macronutrients and
micronutrients
Most of the plant is formed from
Hydrogen, Carbon and Oxygen (~95% of the dry mass).
Carbon comes from carbon dioxide (CO2) in the air.
Hydrogen and Oxygen come from water. Note that this
Oxygen must be available 'mixed in the water', as
Dissolved Oxygen.
The remaining macronutrients,
Nitrogen, Phosphorus, Potassium, Calcium, Magnesium and
Sulfur must be available to the plants root-hairs from
the soil or from fertilizers, as part of the solution
the plants roots are in contact with. Same applies to
the micronutrients, Iron, Manganese, Boron, Copper,
Zinc, Molybdenum and Chlorine.
These essential
elements are mostly used by the plants in ionic form, as
inorganic salts that have dissolved into the nutrient
solution.
Next we will follow the course of an
water drop with some fertilizers in it through the
plant, to learn how plants metabolism works.
The solution in the root-zone
Whether
plants are grown in soil, rockwool or water, solution
with dissolved nutrients must come into contact with the
plants roots. This nutrient solution should be of the
suitable temperature, concentration, acidity and
chemical composition to be healthy and to contribute
positively to the plants growth and well-being.
For 'our-favorite-plant' temperatures should be
between 16-26 C degrees, or 60-80 F degrees. Low
temperatures slow down the metabolism of the plant and
its growth. On the other hand, in high temperatures
there will be less of Dissolved Oxygen in the solution,
causing the roots to be more vulnerable to diseases and
pathogens.
Acidity in the root zone effects
the intake of nutrient ions. Generally for hydroponic
applications the recommended pH range for our favorite
is between pH 5.2 and pH 6.0. If the the nutrient
solution should become more acidic or alkaline then the
availability of certain nutrients would decrease, making
nutrients less available or even completely unavailable
to the plant. Also problems like nutrient ions
precipitating out of the solution could arise.
The concentration of the nutrient solution
should not be too strong, ie. over 1300-1500 ppm, nor
should it be too weak. A strong solution would cause
negative osmotic pressure on the plant. Because of high
salinity, ie. the amount of dissolved solids outside the
plants root cells, water flow would reverse to flow out
of the plants, causing plants to lose their turgor, to
wilt. Too weak solution wouldn't contain enough
nutrients and might cause osmotic flow of nutrients to
reverse, causing nutrient ions to flow out of the cells,
leaving the plant hungry for more.
"If a cell is in contact with a solution of lower water
concentration than its own contents, then water leaves
the cell by osmosis, through the cell membrane. Water is
lost first from the cytoplasm, then the vacuole through
the tonoplast. The living contents of the cell contracts
and eventually pulls away from the cell wall and
shrinks, this is known as Plasmolysis."
Quote from CourseworkHelp: AT1- Osmosis In
potatoes.
Chemical composition of the nutrient solution is
likewise important. Without certain nutrients plants
cannot live, or cannot complete their life cycle. Toxic
substances in the solution could cause the plant to die,
or perhaps cause the grower, enjoying the fruits of
his/her labors, to fall sick or die. Sufficient
Dissolved oxygen levels should be present in the
solution, root-cells need this to breathe, like fish,
underwater. Also the essential elements should be in a
such a form as to be available to the plant, as
inorganic ions. With the plethora of nutrient products
currently available to most growers, the nutrient
composition is rarely a problem.
Rosa Root
hair meets Wally Waterdrop
To simplify, plants
roots are basically composed of surface cells that
absorb the water and the elements, and of inner
structures of veins that translocate the water &
elements, called nutrient solution from here on, upwards
to the stem.
The cells on the root surfaces,
called root hairs because of their 'fuzzy' nature, can
passively diffuse the nutrient solution, or expend
energy and actively transport water and nutrient ions
across their cell membrane.
The
Cell
Every organism on our planet, according to
the science is composed of one or more cells. An average
human might have billions of cells. On the other hand,
bacteria are single celled organisms. Plants are
multicelled, of course. Cells always have an cell wall,
surface membrane, and internal organs.
Cell
wall
The cell wall, often called the primary
cell wall serves to protect the cell from the
surrounding environment and to support the cell. The
primary cell walls of plants are made of tiny cellulose
fibers intertwining on the surface of the cell, pumped
out by tiny cellulose rosettes moving across the surface
of the cells plasma membrane right 'beneath' the cell
wall.
"If you put a plant cell in water, water enters by
Osmosis, then swells up. However, the cell will not
burst. This is due to the fact that the cell walls are
made from cellulose, which is extremely strong.
Eventually, the cell stops swelling, and when this point
is reached, we say the cell is turgid. This is
important, because it makes plant stems strong and
upright."
Quote from CourseworkHelp:
AT1- Osmosis In Potatoes.
Plasma membrane
The surface membrane is
also called plasma-membrane or double lipid
layer membrane, and the internal organs the
cytoplasm. The cell membrane inside the outer
primary cell wall is an complex, living tissue of
biochemical wonders and little molecular machines that
can move molecules back and forth across the membrane
and build the cell wall. There are also little conduits
between the adjacent individual cells, to make transport
of water and ions even easier. These pores are called
plasmodesmata.
Functions of the
membrane
This plasma membrane has many
functions, each function covered by particular tiny
organs, made of proteins:
Keeping the solution balance suitable in- and
outside the cell. There are proteins on the membrane
that can pump water and ions in and out of the cell
wall. It's also referred to with an really advanced term
'Maintaining ionic homeostasis'. Be sure to dazzle your
friends with this term.
Signaling and sensing the environment. Such as
receiving hormonal messages.
Building the primary cell wall. Small organs moving
on the membrane spewing out long strands of cellulose
that form the external cell wall matrix.
Regulating the turgidity. Adjusting the osmotic
pressure.
Communicating with the adjacent cells, through the
plasmodesmata mentioned earlier.
So once an
root-hair-cell starts to feel a little thirsty, or
perhaps gets an message from its neighbor to move in
more nitrogen, it can utilize several strategies to
'transport' the required molecyles from the nutrient
solution, into the cell and onwards. If no energy is
required upon the cells part, this is called passive
transport, and, logically, if energy is expended,
active transport is in progress.
Passive Transport
Because of the
physical and chemical nature of the nutrients ions, the
substances dissolved in the nutrient solution, all the
substances and even the solution itself are subject to
osmosis, diffusion through the selectively
permeable plasma membrane. This is because each molecule
has an electric charge, and differing concentrations of
the molecules create electric potential between the
differing concentration areas, called gradients
(concentration gradient, potential gradient,
trans-membrane electrochemical gradient...).
What is diffusion?
In diffusion
solutes (molecyles) seek to move from the stronger
concentration towards the more diluted thus equalizing
any possible differences in the concentration.
In other words, diffusion is the effect of
molecyles dissolved in solution, diffusing from the area
of higher concentration towards the area of lower
concentration of dilutes.
Suppose two solutions
are mixed in an container: water and pH down. Right
after mixing, concentrations of pH down in the water are
uneven. After a while, after diffusion, pH down will be
equally concentrated across the volume of the water.
Diffusion occurs in solutions consisting of
particles. The energy to diffusion is created from the
random thermal motion of molecyles, also called
the brownian motion.
Diffusion happens
through cell walls also, except where blocked by the
selectively permeable cell wall.
Diffusion
through an cell wall
Anything will permeate the
double lipid layer of the cell wall given enough time.
However, there are large differences in the time period
required.
High permeability (through cell
walls)
Water
Urea
Glycerol
Tryptofan
Glucose
Cl+ (Chlorine ion)
K- (Potassium
anion)
NA+ (Natrium ion)
Low permeability
Table 1. Permeability for some substances
Higher the permeability, the faster is the movement
through the cell walls into the cells.
What
is Osmosis?
Well, Osmosis is actually diffusion
of water with an permeable layer of some kind
that's permeable by the solution. In cell biology terms
words, Osmosis is what diffusion of water through the
cell wall is actually called.
To give an more
practical example, Osmosis is the diffusion of water
from a hypotonic solution, solution that is low in
dissolved solids, into a hypertonic solution which
contains higher amount of dissolved solids across and
selectively permeable membrane.
Osmotic
diffusion through cell walls is passive transport
mechanism, because it requires no energy from the cell's
part.
As you can see above, cell walls can
permeate water and some molecyles easily. However, some
of the molecyles require active effort from the cells to
transport into the cells. This is called active
transport.
What is Reverse Osmosis?
Reverse Osmosis-term is most often used of water
purification systems that use water-permeable layer to
purify water. Reverse Osmosis water contains only water
molecyles (H2O) or molecyles smaller than that. Reverse
osmosis -layers are capable of rejecting bacteria,
salts, sugars, proteins, particles and dyes among other
things (molecule size smaller than ~200 daltons).
In plants the condition of Reverse Osmosis
suggests that the concentration of solutes in solution
outside the (root) cells is higher than inside the
(root) cells and thus the direction of the water
movement is out of the (root) cells, and not inwards.
Simply put, the salty solution draws water from the
plants, often causing plants to wilt.
Active
Transport
The root hair cells can utilize the
transport proteins and ion pumps, located
on the plasma membrane, to actively move solutes across
the membrane. This way plants can control the intake of
water and nutrients from the solution that is in contact
with the root hairs.
There is much more to the
whole transport-business. To learn more about the issue,
type some of these keywords into your favorite search
engine: "cell wall transport active facilitated
diffusion cytosis ATPase".
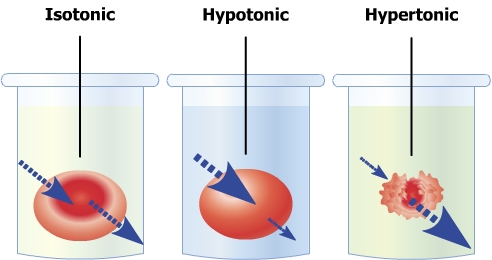
drawing by:
ReSoNiC420
Normal 'hypertonic'
situation
Normally all plants cells are filled
with water, and the whole plant is 'rigid' with the
water. This is caused by the high positive internal
osmotic pressure, also called turgor. This state
of high internal pressure in cells is called
hypotonic. Should a plant lose its turgor, it
would wilt and its leaves would be completely limp. This
opposite state would be hypertonic, ie. when a
cell would have an negative internal osmotic pressure,
causing water to flow out and the cell to shrink (or in
case of rigid-walled cells, the interal cell membrane
(plasma membrane) to shrink).
Most energy for
keeping the cells hypertonic results from the
transpiration, the evaporative pull resulting
from water evaporated through the stomata, small
openings on the lower leaf surfaces, and from the
cohesion & capillary action of the
water in the plants veins (xylem).
Roots
control the environment and intake and exhaust of
solutes Some of the pressure is created actively by
the root-hair cells - cells pump water inside the plant,
using their cellular energy (ATP). In similar
fashion plant can actively transport nutrients, like
mentioned above.
Note that the above is simply
one theory to explain the phenomena that happens in
plants and cells. There are different theories on how
cell-walls, diffusion etc work. For more info on this
theory, do an web search with 'Donnan equilibria'.
Root structure
Roots are responsible
for extracting water and the nutrient minerals from the
growing medium. The root tip, also called apical
meristem grows into the medium, pushing through it
covered by the root cap, an protective shield of
cells.
On the roots surface layer, the
epidermis, root hairs have developed on
top of the cortex, which in turn is formed around
the internal layer of the roots, also called the
endodermis. Root hairs have large surface area
which effectively absorbs nutrients from the medium.
Symbiotic, mycorrhizal fungi can also increase
the surface area, greatly enhancing the intake of
nutrients.
Root hairs
Root hairs
cover the mature root surface. They are tiny hair-like
structures that grow right into the medium and increase
the surface area of the root to asphyxiating numbers.
There can be more than 20000 root hairs on an area equal
to fingernail. On the average length of 5 mm, the
surface area of these root hairs would exceed 1/3 square
meter, over 3 square feet!!!(h=0,005m, r=0,0005m) So
thanks to this huge surface area, roots can supply water
and nutrients to an very large plant.
Root hairs are
often visible by the naked eye. Root hairs are quite
short lived and often mature roots have no visible root
hairs.
Nutrient movement across membranes
The nutrients, minerals dissolved into
water-solution, are transported as ions. Ions are
soluble in water but cannot cross membranes without the
presence of transport proteins, little organs on
the surface of the membrane. The transport of the
negatively charged ions requires the transport of an
positively charged particle in the opposite direction.
These positively charged particles are protons,
H+ - hydrogen without an electron. This way the
electric potential and chemical potential
stays in equilibria, with equal electric
potentials on both sides of the membrane. These protons
are pumped actively across the membrane using
ATP, adenosine triphosphate, as an energy source.
There are basically three mechanisms that
transport the nutrient ions: primary and secondary ion
pumps and ion channels. These are proteins that sit in
the plasma membrane, each type specific to the nutrients
they carry.
Some of the ion pumps move
the H+ protons out of the cell, an some into the cell.
These are known as primary ion pumps. This
movement of H+ changes the potential/gradient, and
facilitates the movement of the other ions. There are
also the secondary ion pumps that move the other
ions in and out of the cell.
Finally there are
the ion channels, little channels with opening
and closing 'gates' that permit the nutrient ions to
move across the membranes, driven by the potential &
electrochemical gradient.
Movement of
nutrient solution inside the plant
Once the
water and the nutrient ions have been absorbed by the
root hair-cells, these are transported across the cell
plasma membranes, directly in the cells,
symplastically, or between the cells, in
intracellular spaces, apoplastically.
Once the solution has traveled through the
root-hairs and the cortex, into the inner parts of the
roots (endodermis), it can only travel into the
vascular system inside the cells, symplastically,
transported through the membranes, in the cells. In the
vascular system, the bundles of veins, there are two
types of veins - the xylem, and the
phloem. A these vascular bundles are
basically vertical veins running from the roots to the
growing tip.
The bulk of the flow is created by
the transpiration pull, drawing the solution
upwards, towards the leaves. Diffusion and active
transport also help in the movement of the solution. The
physical properties of water, cohesion ie. the
attraction of water molecules to one another and the
resulting capillary action also helps in creating the
strong vertical upward movement of water. This is an
very efficient system - plants can move large volumes of
nutrient solution from the roots up to the foliage often
very high above the root level.
Leaving
leaves
The vascular bundles run throughout the
plants, in the stems and the leaves. You can actually
see the bundles in the leaves - the veins of the
leaves. Once aboard the 'plants internal transport
system', the solution is moved around the plant, and the
nutrients used for building blocks, to create energy in
the photosynthetic process, and to regulate the
metabolism and the turgidity of the plant.
Most
of the water is transported into leaves, where it is
evaporated through small openings on the lower surfaces
of leaves. These openings are called stomata
(singular stoma). Plants can open and close these to
control the amount of evaporation. As water evaporates,
it contributes to the total transpiration pull. In
nature the evaporated water floats in the air, condenses
into clouds, rains down on the plants and the cycle is
completed.
How does all this apply to
Cannabis -plants?
So how do the plants roots, or
the roothairs in them, control the nutrient intake?!?
Wouldn't any and all nutrient ions diffuse themselves
all around the plant and the nutrient solution (as
opposed to Nitrogen going to leaves and Kalium to the
stems)?!?
With active transport-mechanisms
root cells can 'select' the ions (and other substances)
that are transported into the cell. This way they can
adjust (to) the environment, and actually even work
against the osmotic imbalance. Looking at the larger
context, plants use the energy from photosynthesis to
keep the juices flowing in the right places.
"Excessive flow of water into a cell by osmosis can
burst the cell. Cells protect against this using
processes of osmoregulation. If external pressure is
applied to the stronger solution, osmosis is arrested.
By this mechanism plant cells can osmoregulate, since
the cell wall of a fully turgid cell exerts pressure on
the solution within the cell." Quote from
CourseworkHelp: AT1- Osmosis In Potatoes.
Nutrient solution and soil management
So
once an grower understands these principles (s)he can
apply these to practice. Its easy to understand that
strong changes in the amount of dissolved substances in
the root-zone would stress the roots by changing the
direction of the osmotic flow. A plant could suddenly
experience strong stress, and possibly even direct
physical damage to the roots.
For each plant
there exists an optimal environment. By measuring the
pH, TDS or EC one can understand the conditions in the
root-zone and act accordingly. The suitable range was
discussed in the second paragraph of this text, The
solution in the root-zone.
DISCLAIMER: Information in this text may
not be completely correct. This text is meant as an
starting point for further study.
|
| Last modified: 04:00 - Jul 08,
2003 |
| |