Contributed by: AeRoGaNiC
Thanks to: aallonharja
Submitted: March 13th,
2005
(aallonharja)
EDTA-compounds are
commonly used in liquid Hydroponic nutrient products to
chelate iron and other metallic nutrient ions.
Chelates
Simply put - chelates
bond to metals the plant uses for growth, in soil or
hydro, and ensures they are stable and mobile. Chelation
is an essential for healthy plants. If chelation is
poor, the plant will be forced to expend energy to find
available nutrients in the medium.
EDTA
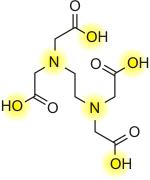
Ethyldiaminetetraacetic acid, also known as
EDTA is used to chelate metal ions. EDTA belongs to a
class of synthetic compounds known as
polyaminocarboxylic acids It has a negative ionic
charge, known as an anion, - EDTA4. The important ions
are highlighted on yellow. These cations bond with
anions to form chelates; ie K+ (potasium anion) will
bond with the EDTA cation.
The ions from EDTA
completely wrap up the entire metal ion all 6 positions.
EDTA bonds really well with Magnesium and Calcium as
well as many other metal ions like Iron. In fact it
appears that Fe is the most finicky element to keep
soluble with pH. EDTA is a great stabilizing agent that
keeps iron soluble in pH fluctuations. The unusual
property of EDTA as a chelating agent is its ability to
chelate ( aka complex metal ions) in 1:1 metal to EDTA
complexes (If one is familiar with the abilities of
chelates, this is a very strong proportion).
Effects on the environment
However, a problem with EDTA is its inability to
biodegrade in the environment. EDTA is found in many
natural waters and occurs at higher levels in
wastewater. This can be a reason to try and avoid EDTA
if you can, its inability to dissolve. Western European
countries have banned the use of EDTA in detergents,
where it is commonly used to chelate metal ions in
tapwater. A ban was adopted in Australia as well.
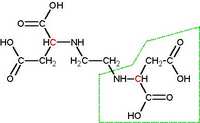
EDDS (S, S'-ethylenediaminedisuccinic
acid), a structural isomer of EDTA, has been used as a
biodegradable substitute. EDDS is a good complexing
agent and is broken down during wastewater treatment
processes, unlike EDTA. This is accomplished by
performing structural change, which can be seen in the
posted pics. The chemical structure of EDDS is organic
where EDTA lacks carbon molecules.
Pic 1: EDTA
Pic 2: EDDS
Organic Chelates
Negative Effects of Synthetic Chelates
EDTA doesnt dissipate in soils and does not
break down. This is a serious environmental hazard. Over
time EDTA can disserve its function. EDTA can cause
reactions in transferable elements and cause
precipitation when it fails. One could even argue this
stress having an effect on yield, and overall
degradation of genetics.
Genetic function relies
upon a perfectly balanced equilibrium of environment and
chelation. Synthetic chelates can, and often do,
interfere with the osmotic equilibrium, by causing
precipitation. This imbalance in the environment can
cause deficiencies, ergo stress. We want to give our
plants the best quality of life. Using organic chelates
have demonstrated in a better efficiency of chelation.
Organic chelates are just more absorbable and stable
than inorganic chelates.
So what the hell can
I use? Humic acid has organic chelates and
chelating agents. The microbial activity greatly
increases the amount of organic chelating agents being
created as well as a natural byproduct. The chelation
agents they produce bond with metal ions and become
available to plants. Chlorophyll is a chelate that
consists of a complex chelating agent with magnesium as
the central atom. Hemoglobin in blood is also a great
chelating agent. Interestingly enough, the only
difference between hemoglobin and chlorophyll is the Fe
central atom as opposed to the Mg atom.
Chelation occurs naturally during composting
though microbial activity. Adding organic material is
also a great way to increase the level of chelating
agents by providing beneficial bacteria with food -
basically increasing their production of natural
chelating agents. Soils with high Cation Exhcange
Capacity are generally high in chelating agents and
organic content.
What are the benefits?
Theoretically less stress. A smooth chelation
process is essential for plants to use minerals and keep
them from bonding with each other, this keeps them
mobile and stops them from precipitating. Chelating
agents nullify the positive charge on the ion and cause
it to be more neutral or be a slightly charged anion,
encouraging the nutrients to transfer through the pores
on the leaf and root surface more rapidly.
Since
pores are negatively charged to attract ions, this
negative or neutral charge makes ionic bonding and
storage of elements in the root pores less likely,
increasing the efficiency of the root system.
Sometimes inorganic elements are bound so
tightly with their chelates (in synthetic situations)
that they cannot be released for physiological function.
The plant has a hard time breaking down the inorganic
chelates, where as the organic chelates do not share any
of the same problems as the synthetics. Using organic
chelates has been demonstrated scientifically to produce
healthier plants.
One thing is for sure, EDTA is
not biodegradable! If you choose to use chemical salts
take some time to see what they are chelated with. Even
in organics, minerals are sometimes chelated with EDTA,
which has been accepted as "organic". However, as I
already said it doesn?t break down, and should be
avoided if at all possible.
Sources
(Chemical Chelates section):
Calculation of EDTA 4 values at any
pH scifun.chem.wisc.edu Ted
Lister, Janet Renshaw :"Understanding Chemistry for
Advanced Level"
F. Albert Cotton :"Advanced
Inorganic Chemistry"
Sources (Organic
Chelates section):
http://www.jhbiotech.com/plant_products/chelation.htm
Bell, Colin F. :"Principles and Applications of
Metal Chelation" Oxford, 1977
foliarfert.com Tro, Nivaldo J.
:"Introductory Chemistry Essentials"
Francis A.
Carey, Richard J. Sundberg :"Advanced Organic Chemistry:
Structure and Mechanisms"
Richard J. Sundberg,
Francis A. Carey :"Advanced Organic Chemistry: Reaction
and Synthesis"